Bringing Flow to the Review and Release Process
The concept of flow is a key element in achieving lean operations. This fact has not gone unnoticed by laboratories but many still struggle to achieve real flow and very often the final review and release of samples can prove to be somewhat of a bottle neck. The final review and release tasks should not be thought of as being autonomous or decoupled from the testing process and should be incorporated in the flowed process.
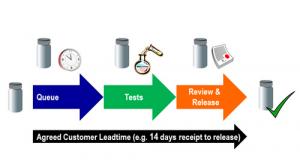
Take the simple representation of the lifecycle of a sample in the laboratory shown in the illustration below. The laboratory will have an agreed customer leadtime for each type of sample (e.g. 14 days for finished goods samples, 30 days for stability samples etc.). This is the maximum time that the laboratory has to perform all the tests, review and release processes. A sample arrives, waits for some time before testing starts, the sample receives all required tests and finally the results receive final review and the sample is released.
Figure 1: Theoretical Sample Flow
In reality, a number of samples will arrive (not necessarily all at the same time) and wait for a period in a queue. Testing is often commenced promptly after sample(s) receipt as there is a perception that if this is to occur, that the sample(s) leadtime will be short. Detailed analysis of leadtime adherence data routinely proves this to be in fact, a misperception. Why is this? The answer is obvious when one looks at the sample flow in more detail.
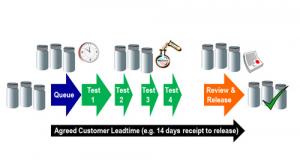
In practice, testing can be very stop-start, or non-flowed as represented by the white space or gaps between arrows in the schematic below. Put another way, the gaps denote waiting periods. Samples can spend a significant amount of time waiting between tests. An even more common phenomenon is that there can be long lag times between the completion of testing and the final review and release. This can lead to backlogs with final review and release and high levels of work in progress (WIP) in the laboratory – something that is both unwelcome for the laboratory and the supply chain. High levels of WIP can mean a lot of effort is expended tracking and prioritizing samples that are partially tested and/or reviewed, and for the supply chain it can lead them to feel that they are not getting a first class level of service from the laboratory.
Figure 2: Common Sample Flow
There is a need to understand the root of the problem and potential reasons for the bottleneck/backlog situation include:
- The review and release tasks are often thought of as being uncoupled from testing. Testing is seen as the main and most important function of the laboratory. But the role of the laboratory is not complete until the final step has been completed – i.e. the final review and release.
- Paperwork pending final review and release is often allowed to build up as resources work on what is perceived to be more important or immediate tasks. This usually stems from the fact that the ‘required by date’ (i.e. in the example above: receipt date + 14 days) has not yet been reached and so there is not necessarily a rush to release. This buffer of time between test completion to ‘required by date’ is itself a product of the practice discussed earlier of starting testing soon after sample(s) receipt, and ultimately leads to workload peaks.
- The choice to employ dedicated reviewers can at first seem an attractive proposition but it can in fact lead to inefficiencies (For a more in-depth discussion than that presented here see the BSM blog: Paperwork Review In QC Labs – Are Dedicated Resources A Good Idea?). Dedicated reviewers can lead to backlogs/inefficient final review and release. There can be peaks and troughs in the review workload, even in a laboratory that is level loading with respect to testing activities, due to the fact that there is variation in testing requirements for different products. If there is a finite number of resources that can complete the final review and release then there is potential for delay of this process. In addition, when dedicated resources are not available (due to training, absenteeism, vacation etc..) there is further potential for the final review and release workload to build up, as it is unlikely that the temporary stand-in can (i) dedicate all their time to review and release and (ii) perform the task in as efficient a manner as they may lack familiarity with the task as it is not their normal function.
The concept of flow can be summarized as follows: “Once you start you don’t stop”. BSM routinely reflects this mantra in the design of flow solutions that are managed effectively with heijunka devices such as rhythm wheels and trains. In fundamental terms, the aim is to eliminate waiting periods between process steps (here tests, review and release). Samples should be allowed to queue just once in the laboratory – that is before they commence testing. Buddy or peer review, real-time review and timely level-loaded final review and release are all mechanisms that can and should be aligned to testing activities to maximize productivity and velocity while minimizing backlogs, workload peaks and the dreaded bottlenecks.
Our consultants can provide further information on the above and discuss any aspect of Real Lean Transformation, simply set-up a call today.