Why are Lab SOPs and Work Instruction so Bad?
Typically, laboratory Standard Operating Procedures and Work Instructions are wordy, patch-worked documents and a hindrance to testing analysts and reviewers alike. Over their life cycle, procedures usually become increasingly difficult to decipher due to multiple disjointed revisions. As a result, training and routine testing often relies on the retained knowledge of key experienced personnel, with an accepted culture of ‘Chinese whispers’. This dependence on undocumented hints and reminders can be tackled by applying Lean thinking to the design and layout of Laboratory SOP’s.
Are SOPs really so bad?
It is not unusual for up to 70% of all LIRs (Laboratory Investigation Reports) and KLEs (Known Laboratory Errors) to fall into the human error category. A significant portion of these can be attributed to method deviations arising from unclear or ambiguous instructions. The primary CAPA (Corrective Action / Preventative Action) for such LIRs and KLEs is an update to the associated test method or standard operating procedure. Where as a pre-emptive strike in terms of “leaning” instructional documents could significantly reduce method deviations.
So, why are SOPs so poor?
Often, SOPs are written by personnel with little or no direct experience of the task being described. This (less than accurate) account is passed along the chain of command for review by a supervisor or manager with even less direct experience. The resultant approved document goes through numerous avoidable revisions throughout its lifecycle as shortfalls are identified by confused end-users. SOP creation and upkeep are rarely scheduled in the same manner as routine laboratory testing and are generally squeezed in after tasks that are perceived as more important.
How to Lean SOPs and Work Instructions
Lean SOPs or Work Instructions provide a higher standard of instructional documentation. The Lean documents are inherently GMP compliant, but are developed with the needs of the end-user in mind rather than just those of the regulatory authorities. A SOP that accurately describes a task to analysts, in a manner easily interpreted by new users, will always satisfy the regulators.
Lean SOPs use photos and diagrams whenever possible, this eliminates subjective descriptions of instruments and machine parts. The benefits of pictures in lieu of verbose descriptions cannot be underestimated. Certain prohibitive practices contribute to the misapplication or omission of graphics in SOPs, e.g. the convention of printing controlled documents on blue paper which can reduce the contrast, the resolution and (by proxy) the value of coloured photographs and diagrams. The typical format of procedures (across the regulated industries) has not been updated since the advent of widespread digital photography. This is a significant missed opportunity.
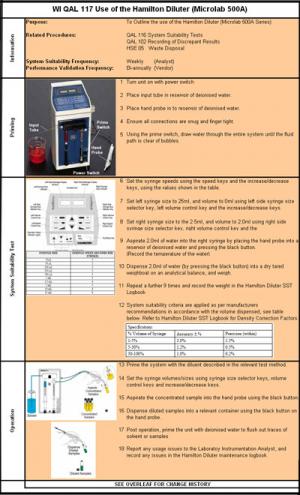
Fig 1 - SOP Before
Fig 2 - SOP After
6 pages of disjointed, confusing text re-engineered to one page of logically sequenced pictures, diagrams and text. (Purpose, scope and change history are documented on the reverse side where they belong!)
Lean SOPs are written by personnel familiar with the task at hand and reviewed by someone unfamiliar with the specific procedure. The review takes place in the form of a “hands on trial”, supervised by the author. Where a step does not provide adequate instruction, an amendment is made. A similar “hands on trial” is carried out on the amended SOP in the presence of the approver (manager), after which time the document can be signed off.
Increased investment in the first draft of a document saves hundreds of hours over its life cycle, in the form of avoided LIRs / KLEs, reduced numbers of revisions and their associated review and approval requirements. Truly Lean instructions speed up the training process, reducing the effort for both trainer and trainee alike.
Our consultants can provide further information on the above and discuss any aspect of Real Lean Transformation, simply set-up a call today.