Lean & Operational Excellence in Life Science CDMO’s (part 1)
Part 1: The Unique Challenges of CDMO Testing Labs
The CDMO sector has experienced significant growth in recent years driven by an ongoing trend in Pharma & Biotech toward outsourcing drug development and production. In 2022, the global Pharma & Biotech CDMO market was estimated at USD 135.85 billion and is expected to continue to grow at a compound annual growth rate (CAGR) of 6.1% between 2023 and 2030.
CDMO’s are typically high complexity, high volatility environments and very different to traditional Pharma & Biotech manufacturing sites. Whereas traditional Pharma & Biotech sites (except for API sites) are often dedicated to one or two products only, CDMO’s typically have to deal with multiple products and relatively short product life-cycles. This key difference means that the significant wastes and opportunities for improvement are also different and unfortunately, the majority of generic Lean and OP Ex initiatives deliver zero or close to zero measurable performance improvement.
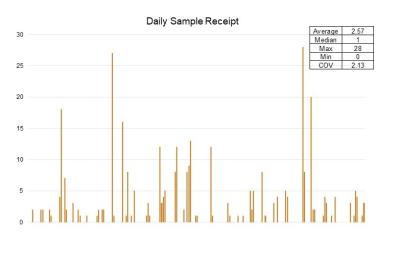
This is especially true in CDMO labs where the short interval volatility is often quite extreme driven by a complex mix of batch / lot release testing and new product project work.
Leveling Workloads: Taming Volatility in CDMO Labs
This type of volatility causes low productivity (during lulls) and/or poor lead time performance (during peaks). Very often the capacity of the lab is not well understood and there is no mechanism to level the workload coming into the lab. If left unchecked this volatility results in the consumption of excess resources and valuable lab space. Lab processes also become stressed leading to constant re-prioritization and ‘stop start’ progress on individual samples or project work. This reduces effectiveness and adds waste. The rate of failures and rework also often increases.
Poor utilization of analyst resources (usually in the form of volatility and imbalance in individual analyst daily workloads) is also quite common.
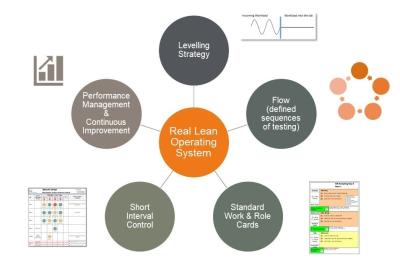
The focus of weaker generic Lean and Operational Excellence initiatives is usually on simple waste elimination and the application of easy to understand tools like 5S, Value Stream Mapping and Kaizen. While these methods may yield some results, we know that more significant and sustainable gains will be realized by applying the deeper Lean concepts of Leveling, Flow, Pull and Standard Work.
In an environment like this, the focus must be on developing a ‘System of Operations’ and Lab processes that effectively level short interval workloads, reduce throughput times (i.e. create flow) and support an appropriate level of standardization of tasks. These new processes are then supported by digital visual management based ‘Short Interval Control’ and ‘Performance Management’ Processes
This leveling, flow and standard work approach also enables the development of balanced, productive and repeatable roles for analysts.
Our consultants can provide further information on the above and discuss any aspect of Real Lean Transformation, simply set-up a call today.