Active Pharmaceutical Ingredient (API)
Objectives
Reduce the overall lead time from manufacture of the product to final release (Quality Control and Quality Assurance).
Solutions Deployed
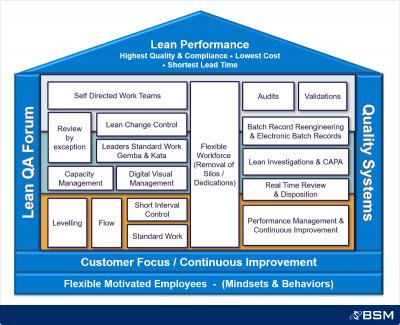
Quality Control: queue based levelling and rhythm wheel, standard work roles, visual management.
Quality Assurance: batch record re-engineering, process optimization (batch record review and corrections, investigation management, disposition).
Results
- Reduction in QC lead time from an average of 13.4 days to an average of 4.7 days
- Alignment of the QA timeline to the QC timeline i.e. disposition ready to complete as soon as the lab results are finalized
- Fixed repeatable analyst requirement
- Continuous improvement culture and mind-set growing within the laboratories