Biologics Drug Substance and Drug Product
Objectives
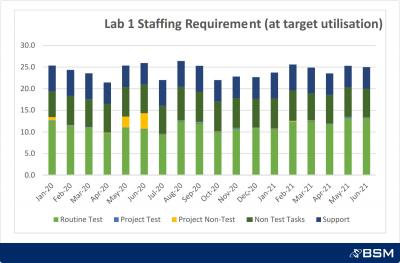
This project was targeted to improve the speed, quality and productivity of the QC functions at individual sites to contribute to a more stable supply of product. Additionally, it focussed on development of a standard capacity planning tool and process to provide visibility of resources at the site and network level.
Solutions Deployed
-
At a Network level: A standard best practice visual management model covering daily process control and medium-term performance management; a consistent capacity planning tool and process
-
At a Site level: Levelling, flow & standard work solutions specific to a variety of lab types; Standard best practice approaches to 5S and consumable management
Results
-
Productivity improvements of the order of 15% released via levelling, flow and standard work
-
Consistent adherence to all lead time targets
-
Accurate projection of required QC resources highlighting likely capacity constraints
-
Implementation of a standard set of global QC KPIs and linking of these to identification and initiation of continuous improvement opportunities
-
Daily lab huddles driven by a standard visual management system