Synchronising Planning, Manufacturing & QC
Day to day operations of individual departments in life science companies rely on many decisions made outside of each department’s own remit. When embarking on a Lean strategy, the pillars of operational excellence (Levelling and Flow) can be supported by increasing awareness of how each department functions and explaining constraints.
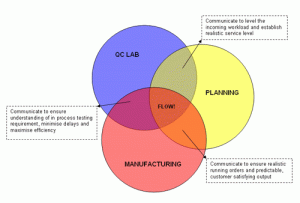
Communication between Planning and QC
A good starting point in rectifying the situation is to quantify the workload associated with each raw material, in-process sample and finished product in terms of QC testing. At the very least, the planning department will no longer plan to use raw materials sooner than they can be tested; at best, the planning department can re-order their requirements to allow the same workload to enter the lab on a daily or weekly basis, enabling levelling, while at the same time satisfying the needs of the manufacturing area.
Communication between Manufacturing and Planning
In a similar situation to QC, manufacturing departments often operate in an unlevel manner. Availability (or unavailability) of raw materials can lead to preventable last minute revisions of manufacturing running orders, typically without consideration for available manpower, vessels or manufacturing durations. Quantifying the manpower required for manufacture provides crucial information to those involved in planning the manufacturing running orders. When cross-referenced against the available manpower, achievable manufacturing plans can be designed. (Note: it is important that the available manpower is aligned to the actual requirements).
Minimising the late revision of manufacturing plans ends the vicious cycle, whereby a manufacturing change requires a lab raw material scheduling change, which has a knock-on effect on the availability of raw materials required further down the plan, leading to further manufacturing plan revisions.
Communication between QC and Manufacturing
Where batch manufacture requires some laboratory input (e.g. in process pH or assay), it is important that the manufacturing department understand the testing workload involved, in terms of system suitability analyses and actual sample testing time. An achievable service level agreement (SLA) should be determined to allow the lab sufficient time to perform the testing, without interfering with on-going analyses, while meeting manufacturing needs. Realistic SLAs allow manufacturing to schedule “filler” tasks to ensure maximum productivity while awaiting results.
Conclusion
Open communication between all three functional areas is imperative while designing optimised lean strategies. With a levelled workload and predictable outputs the task of “flowing” the entire process is simplified and with increased transparency further opportunities can be identified.
Our consultants can provide further information on the above and discuss any aspect of Real Lean Transformation, simply set-up a call today.