Lean Thinking for Laboratories
While it might sound like some sort of fad diet, “lean” in the context of business improvement refers to a specific methodology that originated in the Japanese motor industry toward the end of the 1980s. Over the decades, this lean philosophy has been successfully adopted by many companies across a broad spectrum of industries and, more recently, lean thinking has filtered into laboratories. The focus of a lean laboratory is to test samples in the most efficient way possible in terms of cost, or speed, or both. Although most of the key principles of lean apply in labs, the specific challenges facing laboratories require significant adaptation of standard lean tools.
Laboratories are typically faced with more volatility and variation in the type and volume of work they are required to perform than are manufacturing operations. The life science industry is faced with the additional layer of GMP and GLP complexity. In this context, attention to efficiency and productivity is often secondary. However, there is in fact no inherent conflict between efficiency and compliance. Lean processes in a laboratory achieve regulatory compliance in the most efficient and productive manner possible. A lean lab welcomes auditors and delights in the opportunity to showcase its process improvements along with its capacity to satisfy not just regulators, but customers (internal and external) too.
The factors affecting laboratory performance
Performance in today’s laboratories tends to be negatively affected by a number of issues. These include:
Volatile incoming workload
Most labs experience a volatile incoming workload, with significant peaks and troughs. This results in low productivity during troughs and/or poor lead-time performance during peaks. Understanding laboratory capacity and resource requirements and leveling workloads in a meaningful way is the crux of “leaning” a laboratory. The surest way to fail is to put more work into the lab than it can handle!
Too much WIP (Work in Progress)
Samples do not “flow.” While laboratory analysts may progress speedily through testing, haphazard scheduling resulting from cross-training deficiencies and instrument conflicts means that, at a given time, many samples are partially tested but few are fully completed. Lean in the laboratory means performing the right test at the right time in order to minimize WIP.
Long and variable lead times
In an attempt to be more productive, many laboratories build up queues of samples for tests with long set-up times (e.g., HPLC or GC). While some grouping of samples is a good idea, this is usually overdone and needs to be controlled in order to avoid long and variable lead times, equipment conflicts, and imbalance in daily analyst workloads.
Ineffective fast-track systems
Labs often attempt to address the unpredictability associated with long and variable lead times by developing a fast-track system to allow urgent samples to be dealt with separately. Typically, however, the proportion of fast-track samples increases over time, to the point of being unmanageable. In designing a lean solution, laboratories can increase the velocity of every sample, eliminating the need to prioritize. This may sound impossible, but it can and should be done. If it is done correctly, the amount of analyst effort required per sample does not change; samples simply spend less time in queues.
Lack of cross-skilling
Training gaps are sometimes attributed to high analyst turnover; however, lack of cross-skilling is often as much of an issue in laboratories with low turnover where staff members have become subject matter experts and are de facto dedicated to specific tests or activities. Labs tend to operate at extreme ends of a spectrum, whereby they are either mimicking Henry Ford–style mass production, with analysts repetitively carrying out the same tasks day in, day out, or they are like “craft producers,” taking a sample through its full testing schedule whether it is productive to do so or not. In most cases, the optimum resourcing will be in the middle, with each analyst having the capability of doing all of the testing, but carrying out defined combinations of tests that use their time well and reproducibly meet demand in the most productive way.
Muda, Mura, Muri: Lean laboratory pitfalls
The focus of many lean laboratory projects is almost totally on waste reduction (or Muda, to give it its Japanese name). Significant time is spent on valuestream mapping to highlight new “wastes” to work on; while some improvements are made, these are often not converted into meaningful overall performance improvement.This is no surprise; waste is easy to see and understand whereas the more challenging wastes of “Mura”—the waste of unevenness (volatile workloads) and “Muri”— the waste of overburden (imbalance in daily analyst workloads) are often ignored. These are more difficult to understand, but effective dealings with them will deliver significantly greater productivity improvement than will tackling waste alone; indeed, this will provide a context in which the benefits of waste reduction can be “spent.”
The recipe for success
The most successful lean laboratory projects tackle the “leveling” and “standard work” aspects first. The best foundation for a lean laboratory is analysis of historical data and forecasts, in order to understand the leveled demand rate of testing and resourcing so as to adequately meet this leveled demand rate. Successful leveling of a volatile load and mix will significantly improve productivity and/or lead time. The productivity improvement can be used to provide additional capacity or converted into a cost reduction.
The results
Depending on the focus of the lean initiative, results will be seen in productivity improvement or increased testing speed— but often results will be seen in both areas.
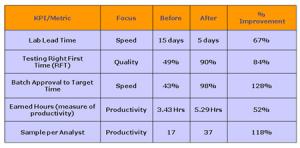
Fig 1: Measurable improvements based on recent lean projects
These measurable improvements (from laboratories that have recently completed lean projects) demonstrate what can be achieved.
Lab work is not the same as manufacturing, but lean thinking can and should be applied in the laboratory environment.
Our consultants can provide further information on the above and discuss any aspect of Real Lean Transformation, simply set-up a call today.